How Many Atoms Does Silver Chromate Have
Therefore we must multiply this 3 by the number of atoms within the parentheses- Oxygen atoms. A sample of carbon dioxide has a mass of 520 g a.

Silver Chromate Formula Molar Mass Study Com
CID 26090 Dichromic acid CID 23954 Silver Dates.
. View the full answer. Chromate 2- is a chromium oxoanion resulting from the removal of two protons from chromic acid. How many carbon atoms are present.
Write down the oxidation half-reaction for the reaction that takes place. 1 x 3 3 atoms. If we take into account we have 2 aluminum atoms in total we will have- 12 oxygen atoms- 3 chromium atoms- 2 aluminum atoms.
Im really sorry I got sidetrackedP This is the question I wanted to ask you In a saturated solution of silver sulfite the concentration of silver ion is 36 x 10. Mass of single silver chromate. A silver ring contains 00134 mmol Ag.
It contains 2 Potassium atoms 1 oxidation state 1 Chromium atom 6 oxidation state and 4 Oxygen atoms -2 oxidation state Is there any way to make lead chromate. How many neon atoms are there in five moles. How many moles does 2501020 atoms Fe contain.
Ions b How many CrO42- ions are present. Since they both contain one mole you have 602 x 10 23 particles of silver and 602 x 10 23 particles of platinum. Salts of chromic acid containing the CrO 2-4 radical.
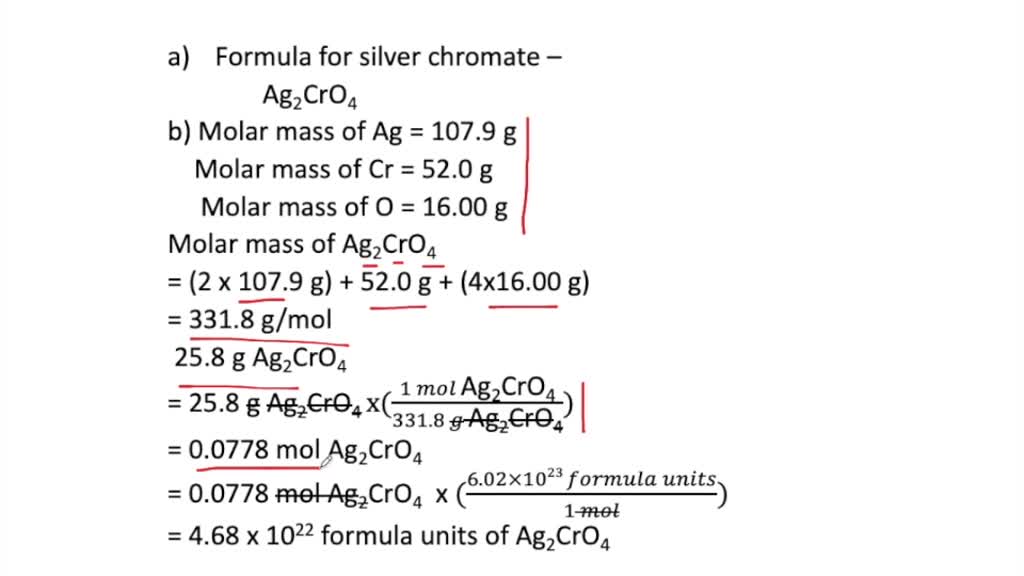
2 AgNO 3 K 2 CrO 4. How many Ag ions are present in sample of silver chromate Ag2CrO4 that has a mass of 258g. Get college assignment help at Smashing Essays Question A sample of silver chromate Ag2CrO4 has a mass of 247 g.
Then silver ions in 00861 moles of silver chromate. It has a role as an oxidising agent. 12 3 2 17 atoms.
It is a conjugate base of a hydrogenchromate. 1 Structures Expand this section. How many moles of silver chromate will be produced from 146 g of silver nitrate.
A How many Ag ions are present. Question A sample of ethanol C2H5OH has a mass of 438 g. When water boils it is still water just in a different state.
The silver comet has silver 80 chromium CR on oxygen. Ions c What is the mass in grams. How many grams of silver chromate will precipitate when 0150 L 0f 0600 M silver nitrate is added to 0100 mL of 0500 M potassium chromate.
Question a buffer solution is made by adding 30 mol of. Silver Ag and chromate CrO4-2 And within the formula Ag2CrO4 there are two silver ions and one chromate ion for a total of three ions. Therefore the molar mass can be calculated as two times the molar mass off 80 plus Mullah Omars off CR plus four times molar mass off oxygen which gives us the molar mass off silver comet as 318 g per mole.
Oh it has two atoms off 81 item off CR and four atoms off. How many silver atoms does it contain. How many chlorine atoms are in 191 moles of carbon tetrachloride.
Question A sample of ethanol C2H5OH has a mass of 438. It is a divalent inorganic anion and a chromium oxoanion. How many atoms are in 064 moles of strontium.
Mass of silver chromate 286 g. 321E22 atoms is how many moles. Solubility and mass are not chemical properties because they dont involve chemical reactions or changing the identity of a substance.
What is the name of the compound Na2CO3. Number of chromate ions. How many atoms are represented by the formula CBr4.
C How many oxygen atoms are present. How many atoms are represented by the formula Li2CO3. 4 x 3 12 atoms- Chromium atoms.
How many ions are represented by the formula Li2CO3. Molar mass of silver chromate 332 gmol. When a zinc strip is placed in a copper nitrate solution the strip becomes coated with copper.
Solution for How many grams of silver chromate will precipitate when 0150 L 0f 0600 M silver nitrate is added to 0100 mL of 0500 M potassium chromate. Determine the number of representative particles in 0150 mol NaCl. Now we have a 3 outside the parenthesis.
How many CrO_42- ions are present in sample of silver chromate Ag2CrO4 that has a mass. A How many carbon atoms does the sample contain. 1 a Given mass of CO252 g Molar mass of CO244 gmole No of moles of CO2Given massmolar mass 52 g44 gmole 1181 mole 1 mole CO2 has 1 m.
Moles of silver chromate 1 mole of silver chromate has 2 mole of silver ions and 1 mole of chromate ions. 2 Names and Identifiers Expand this section. B How many hydrogen atoms are present.
The difference would be in the number of protons neutrons and electrons present because silver contains 47 protons per atom 47 electrons per atom and 61 neutrons per atom while platinum contains 78 protons per atom 78 electrons per atom and 117 neutrons.

The Mole Chapter Ppt Video Online Download
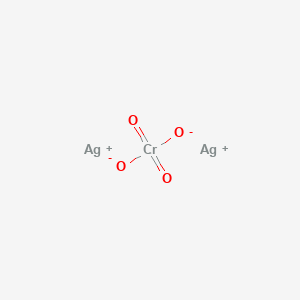
Silver Chromate Ag2cro4 Pubchem

Solved Challenge A Sample Of Silver Chromate Has A Mass Of 25 8 G A Write The Formula For Silver Chromate B How Many Cations Are Present In The Sample C How Many Anions

Solved A Sample Of Silver Chromate Has A Mass Of 25 8 G Begin Equation Begin Array L Text A Write The Formula For Silver Chromate Text B How Many Cations Are Present In The
0 Response to "How Many Atoms Does Silver Chromate Have"
Post a Comment